Health
U.S. FDA APPROVES PRODUCT FOR INJECTION INTO THE LIPS FOR ADULTS OVER AGE 21
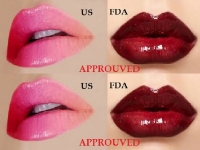
THE ONLY DERMAL FILLER PROVEN RESULTS

(Source: (Photos) Socialife Chicago - Youtube)
USPA NEWS -
Allergan Plc, a leading global pharmaceutical company, announced October 1, 2015, that they have received approval from the U.S. Food and Drug Administration to market JUVEDERM® ULTRA XC for injection into the lips and perioral area for lip augmentation in adults over the age of 21...
Allergan Plc, a leading global pharmaceutical company, announced October 1, 2015, that they have received approval from the U.S. Food and Drug Administration to market JUVEDERM® ULTRA XC for injection into the lips and perioral area for lip augmentation in adults over the age of 21. It is the only dermal filler that has proven results lasting up to a year for lip augmentation.
Allergan Plc is focussed on developing, manufacturing and commercializing innovative branded pharmaceuticals, high-quality generic and over-the-counter medicines and biologic products for patients around the world. Allergan markets a portfolio of products that provide treatments for the central nervous system, eye care, medical aesthetics, gastroenterology, women's health, urology, cardiovascular and anti-infective therapeutic categories.
Important safety informations :
The product should not be used in patients who have severe allergies, marked by history of anaphylaxis or history or presence of multiple severe allergies, and should not be used in patients with history of allergies to gram-positive bacterial proteins or lidocaine.
Some of the precautions to be taken :
- In order to minimize the risk of potential complications, these products should only be used by healthcare professionals who have appropriate training, experience, and knowledge of facial anatomy.
The product should not be used in patients who have severe allergies, marked by history of anaphylaxis or history or presence of multiple severe allergies, and should not be used in patients with history of allergies to gram-positive bacterial proteins or lidocaine.
Some of the precautions to be taken :
- In order to minimize the risk of potential complications, these products should only be used by healthcare professionals who have appropriate training, experience, and knowledge of facial anatomy.
- Healthcare professionals are encouraged to discuss the potential risks of soft-tissue injections with their patients prior to treatment and ensure that patients are aware of signs and symptoms of potential complications.
- The safety for use during pregnancy, in breastfeeding females, and in patients with known susceptibility to keloid formation, hypertrophic scarring, and pigmentation disorders has not been studied.
- Patients who are using products that can prolong bleeding (such as aspirin, nonsteroidal anti-inflammatory drugs, and warfarin) may experience increased bruising or bleeding at treatment sites.
- The safety for use during pregnancy, in breastfeeding females, and in patients with known susceptibility to keloid formation, hypertrophic scarring, and pigmentation disorders has not been studied.
- Patients who are using products that can prolong bleeding (such as aspirin, nonsteroidal anti-inflammatory drugs, and warfarin) may experience increased bruising or bleeding at treatment sites.
Ruby Bird Yasmina Beddou U.s. Fda Product Injection Lips Adults Age 21 Dermal Proven Results Allergan Plc Pharmaceutical Juvederm Ultra Xc Lip Augmentation High-quality Generic
Liability for this article lies with the author, who also holds the copyright. Editorial content from USPA may be quoted on other websites as long as the quote comprises no more than 5% of the entire text, is marked as such and the source is named (via hyperlink).